Nano particle size tungsten trioxide for preparing thermal insulating glass may be prepared by hydrothermal reaction using sodium tungstate, urea and hydrochloric acid as raw materials. The hydrolysis of urea under hydrothermal conditions releases NH4+ and HCO3-. It was found that the cations (NH4+) can stabilize the metastable hexagonal phase tungsten trioxide. Then, (NH4)2SO4, which has an equivalent molar amount to NH4+ of urea, and NH4HCO3, which has an equivalent molar amount to HCO3- of urea, are respectively added to the reaction system instead of urea. The synthesized samples were subjected to XRD test, and the results are shown in the following image.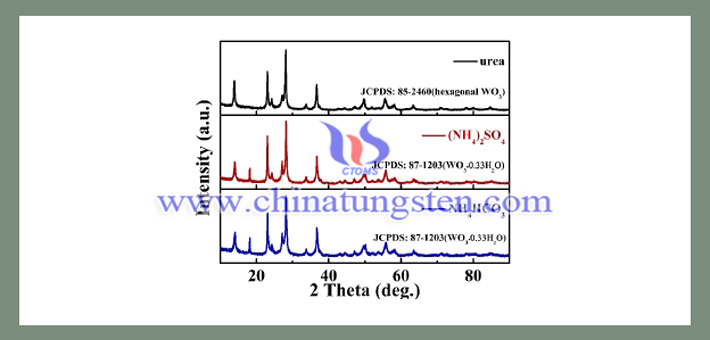
From the XRD results, it was found that the two samples synthesized were orthogonal phase hydrated tungsten trioxide. In the synthesis of hexagonal phase tungsten trioxide, an excess of monovalent cations needs to be added. And it was found that the amount of monovalent cations added in the replacement experiment was insufficient to form hexagonal tungsten trioxide, and the equimolar amount of HCO3- did not affect the formation of hexagonal tungsten trioxide. However, the addition of urea completely changed the experimental results. In general, the release of NH4+ and HCO3- from urea is a slow process, while the replacement of (NH4)2SO4 and NH4HCO3 can result in the immediate presence of NH4+ and HCO3- in the system. This is the difference between the addition of urea and (NH4)2SO4 and NH4HCO3. Therefore, it can be seen from the results that the slow release of the monovalent cation enables the metastable hexagonal phase to be stably formed during nucleation and growth although the concentration of the monovalent cation is insufficient.
More details, please visit:
http://www.tungsten-powder.com/Tungsten-Trioxide.html




