Nano particle size tungsten trioxide for manufacturing ceramic microsphere may be prepared by hydrothermal reaction using Na2WO4?2H2O, urea and concentrated hydrochloric acid as raw materials. In this process, the NH4+ produced by urea decomposition makes the metastable hexagonal phase tungsten trioxide stable. Therefore, it is necessary to study the effect of urea on the crystal structure of tungsten trioxide after stirring at room temperature under hydrothermal treatment.
After the addition of urea, the solution was stirred at room temperature for 24h, and the obtianed mixture was placed in a hydrothermal kettle, then reacted at 140°C for 24h. The obtained sample was subjected to XRD test, and the results are shown in the following pattern.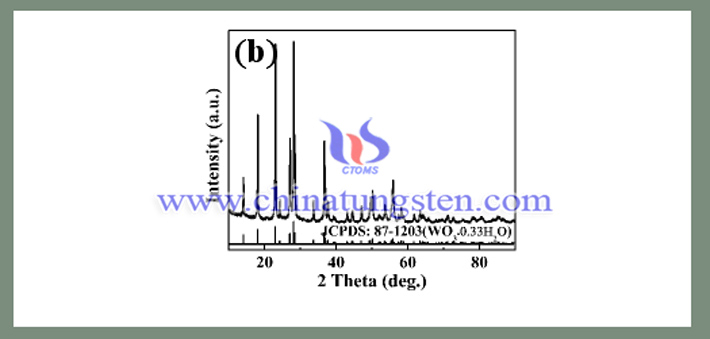
Compared with the standard card, it was found that the obtained sample is orthogonal phase hydrated tungsten trioxide. It indicates that the tungstic acid undergoes a dehydration process under hydrothermal conditions to obtain hydrated tungsten trioxide. And it cannot be converted to hexagonal phase tungsten trioxide by hydrothermal process. Since the hydrolysis of urea at room temperature is very slow, the urea does not affect the crystal structure of the sample at room temperature. After the precursor has been formed, the decomposition of urea under hydrothermal condition will not affect the crystal structure of the sample. Therefore, from the above results, it is known that the slow decomposition of urea plays a key role in the nucleation and growth of the hexagonal phase tungsten trioxide.
More details, please visit:
http://www.tungsten-powder.com/Tungsten-Trioxide.html




